FDA Approves Vaccine, Distribution Imminent
December 12, 2020
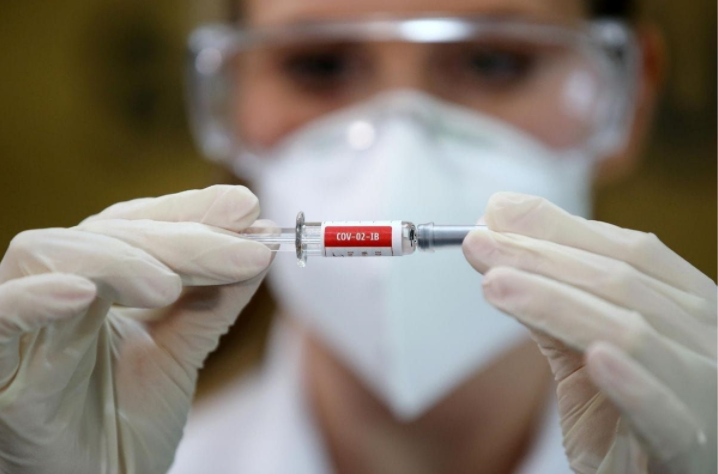
Friday the US Food and Drug Administration issued its official stamp of approval to Pfizer, the company responsible for the development of a COVID-19 vaccine.
This marks a crucial turning point in a nearly year-long fight with a global pandemic that has already taken close to 300,000 lives in the US alone.
Pfizer’s vaccine, being the first such treatment to gain the green light from the FDA for emergency distribution in the US, joins a handful of other vaccines set for immediate deployment across the globe, most notably Europe’s AstraZeneca and Sputnik V vaccines, which are still awaiting official confirmation.
Vaccines typically take years to develop.
However, thanks to a trans-national effort to fund, staff and support vaccine development, Pfizer’s breakthrough only took a miraculous eleven months.
Operation Warp Speed, the US government’s multi-billion dollar vaccine research program, will oversee a goal of distributing up to 100 million doses by March.
Over the next week, 2.9 million vials are set to be sent across the country, targeting America’s frontline communities—healthcare workers and nursing home patients.
Set in motion by the Trump administration and confirmed by President-elect Biden, all shots will be conducted free of charge.
Needless to say, the news of a COVID-19 vaccine is cause for both rejoicing and logistical panic for federal, state and local governments alike.
With a limited quantity of doses available and demand, understandably, at an all-time high, governmental officials are put in the unenviable position of pursuing preferential distribution.
Hospitals in many parts of the country are already under immense strain, pushing the limits of understaffed clinics and hospitals to the brink.
Effective distribution of a vaccine will take an all-hands-on-deck approach on both the federal and state levels.
Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Disease, whose duties will be renewed by the Biden administration come January, has committed to a policy of vaccinating all healthcare workers, high-risk individuals, teachers, childcare workers, and persons of “national importance,” in that graded order.
“We’re fairly confident that anyone who wants the vaccine, who is categorized as one of those front-line clinical care team members, will be able to get the vaccine when we have it available to distribute,” explained Dr. Jeff Bahr, the man responsible for overseeing medical group operations for the Advocate Aurora Health System in Wisconsin.
The process of researching, developing and distributing such a vaccine is “almost like building an airplane while you’re flying it,” he continued.
“You’re accumulating data on people real time and getting it to the people in the labs real time so that they can get to work. Never before in human history have we seen a turnaround time like this,” Bahr said.
With the new year fast approaching, all eyes are focused on the end of the COVID-19 pandemic, and today is a strong start.





































Becky Bain • Dec 17, 2020 at 11:09 am
Awesome reporting, Nick!